

Following the gradual update of its entire product line's outer packaging starting October 1st, TLPH(Lao) recently announced that it will carry out a step-by-step, batch-by-batch update and optimization of the coating materials used for its current tablet products to enhance product quality to a higher standard.
The first batch of updated products, Lorlatini(Lorlatinib) and Elobopa(Eltrombopag), which have been approved for filing by the Lao Ministry of Health's Food and Drug Department (FDD), have now entered the market.
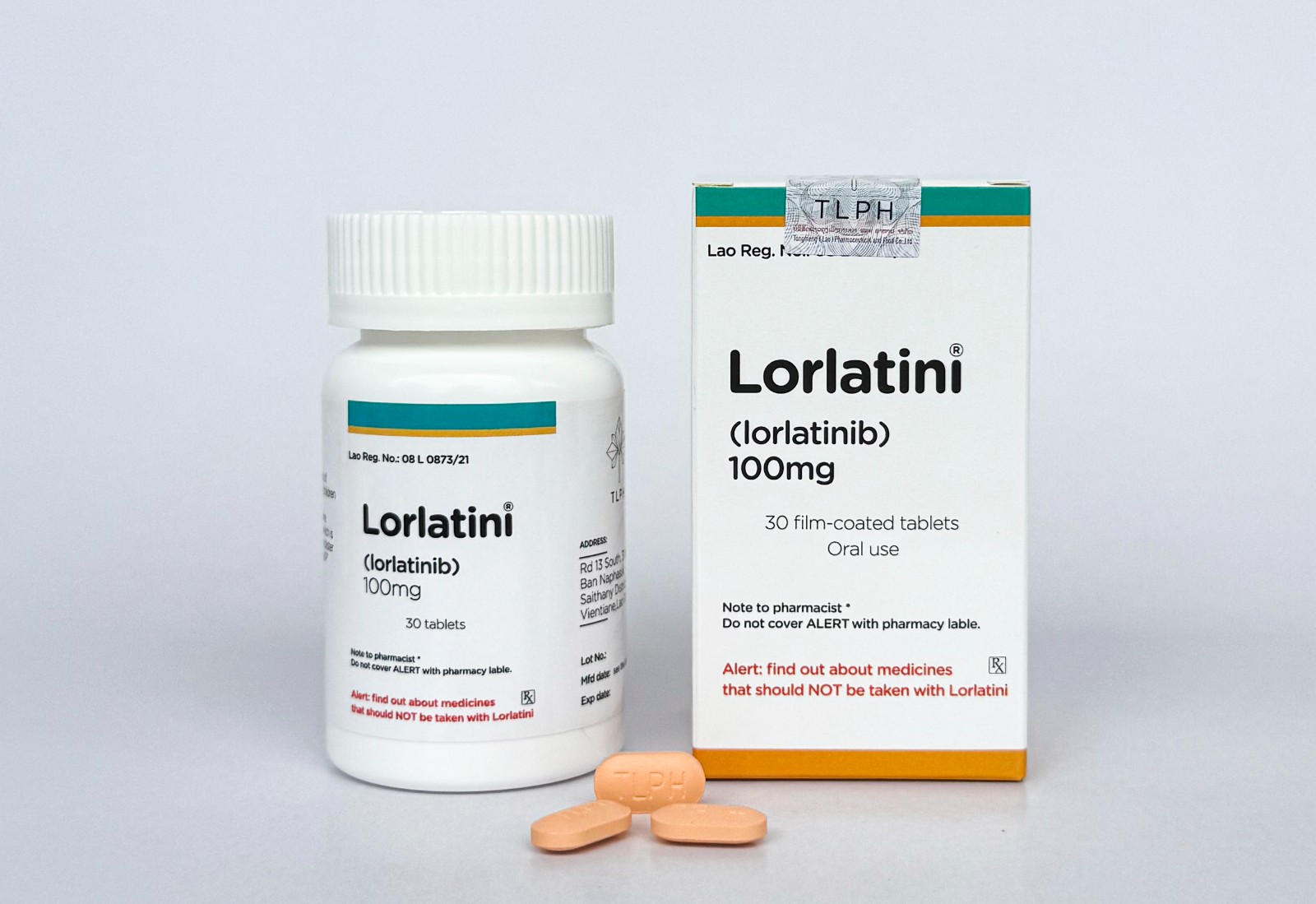
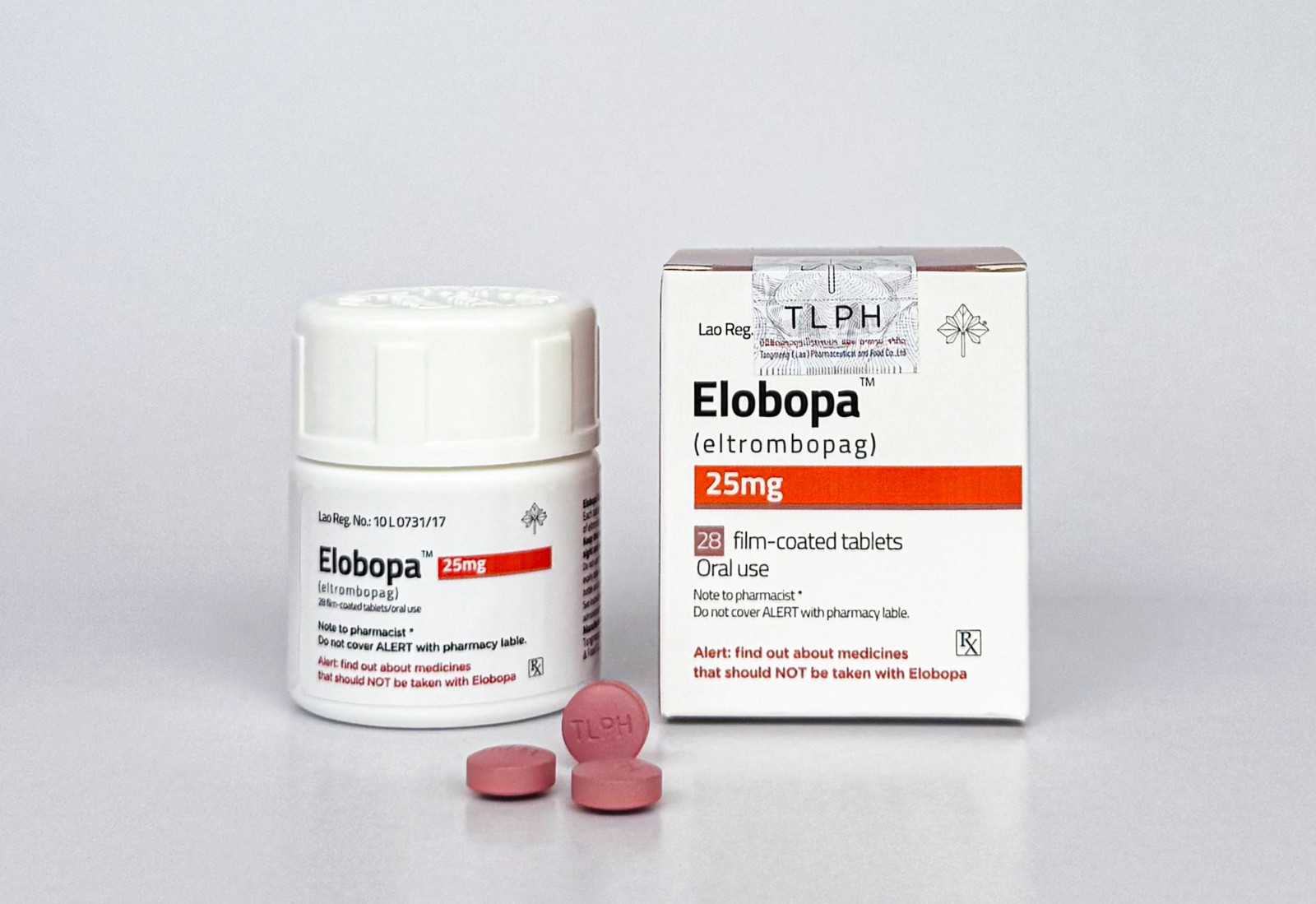
In this regard, TLPH(Lao) solemnly declares that both the new and old batches of products are legal and authentic pharmaceuticals. The only difference lies in the color of the tablet coating, with no other variations. Consumers can purchase and take them with confidence!